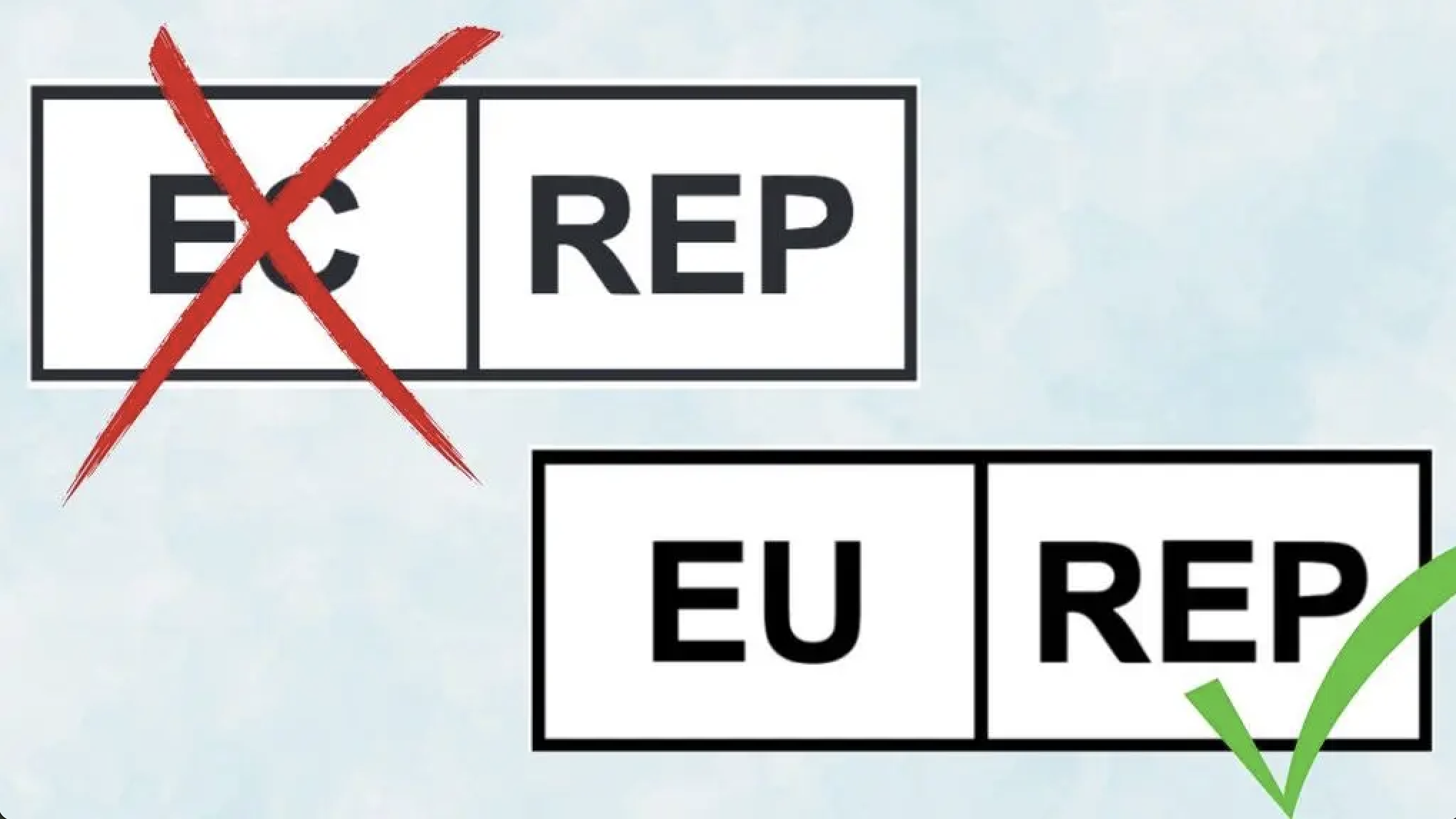
Even a small change to a labeling symbol can ripple across global operations, causing serious delays and risking costly recalls. With the newly announced shift from “EC REP” to “EU REP” as outlined in the latest amendment to ISO 15223-1, it’s important to get started on implementing mass changes as soon as possible to maintain compliance and prevent reputational damage. So, what does this latest regulation update mean for your business, and how can you stay ahead?
What’s Changing with ISO 15223-1?
The ISO 15223-1:2021+Amd 1:2024 standard introduces a key update: the traditional "EC REP" symbol used to indicate an authorised representative in the European Community is being phased out in favour of a more regionally adaptable “XX-REP” format.
For manufacturers selling into Europe, this means adopting the “EU REP” symbol to represent their European Union Authorised Representative. Other variations like “CH REP” (Switzerland) and “UK REP” (United Kingdom) are also emerging in local jurisdictions.
This change reflects today’s political borders and helps create a more adaptable approach to labeling in different regions as well as eliminating any confusion with the symbols. You can read more about the update here.
Why It Matters
While this may seem like a minor design tweak, the impact is significant—especially for manufacturers operating across the world. With every single use of the “EC REP” symbol needing to be identified, replaced and re-approved, companies often turn to expensive third parties to make these changes. With proper end-to-end labeling and artwork management software that can automate these changes, the process can be quick, simple and smooth, all while keeping costs down.
Beth Peckover, Chief Delivery Officer at Kallik explains: “While comprehensive labeling software may seem like a significant upfront investment, the ability to instantly implement thousands of updates in response to evolving regulations can save businesses enormous time, cost, and compliance risk in the long run. Regulations are constantly changing–and fast–meaning automation isn’t just a nice-to-have, it’s essential for staying competitive and audit-ready.”
How Kallik Can Help
At Kallik, we help medical device and pharmaceutical companies stay ahead of regulatory changes with powerful automation. With Veraciti™, you can update 10,000 labels in just 14 days—compared to six months using traditional methods—thanks to our smart, automated approach.
Our ‘Where Used’ function instantly identifies where a specific symbol or warning appears across all artworks and labels, enabling fast, accurate updates. Veraciti also flags layout issues, handles size variations across product ranges, and ensures changes don’t compromise compliance.
By replacing manual processes with cloud-based automation, customers have seen up to a 70% reduction in artwork costs. Tools like Automated Artwork Generation and Cascade maintain brand consistency and allow seamless integration with design tools like Adobe InDesign. Veraciti even automates approval workflows, cutting delays and accelerating time to market.

Future-Proofing Your Labeling Strategy
The update to ISO 15223-1 isn’t an isolated change—it’s part of a broader trend toward greater localization and increasing regulatory divergence across global markets. The emergence of country-specific reps and corresponding symbols (EU REP, UK REP, CH REP, NZ REP) only adds to the complexity.
This makes it more important than ever to modernize your labeling systems. Manual processes and fragmented artwork files simply won’t scale in a world where regulatory updates can happen any time, anywhere. With Kallik, your business is ready not just for today’s changes, but for whatever comes next.
Ready to Simplify Your Labeling Compliance
Whether you're preparing to update your EU REP symbol or facing broader challenges with global labeling requirements, Kallik can help. Speak to one of our labeling and artwork experts to learn more about how Veraciti™ can help your business thrive in a rapidly changing regulatory environment. Get in touch today at enquiries@kallik.com or call +44 (0) 1827 318100.
